

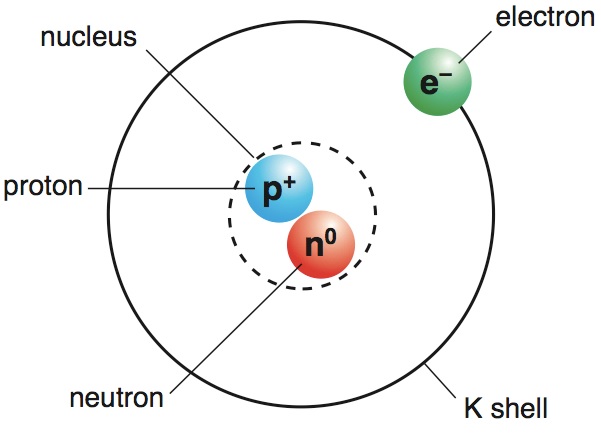
© 2006 University of Chicago Library Descriptive Summary Title: Bohr’s Atomic Model: Accessed 19th April 2022.University of Chicago Library Guide to the Niels Bohr Collection 1909-1963.Bohr Model of Atom: Accessed 19th April 2022.Bohr’s Model of an Atom: 19th April 2022.We hope you enjoyed studying this lesson and learned something cool about Bohr’s Atomic Model! Join our Discord community to get any questions you may have answered and to engage with other students just like you! We promise, it makes studying much more fun!😎 REFERENCE The atom is depicted as an atomic nucleus having protons and neutrons, with electrons in circular orbitals at particular distances from the nucleus, according to the Bohr model. Niels Bohr offered a theory for the hydrogen atom in 1913, based on quantum theory, which states that some physical quantities have only discrete values. How do electrons move in Bohr’s model?Įlectrons in Bohr atomic model travel in prescribed circular orbits around the nucleus. Bohr found that electrons far from the core have more energy than electrons near the core. In Bohr's concept, a small (positively charged) nucleus is surrounded by negative electrons that move in orbit around the nucleus. By generating or absorbing energy, electrons can leap from one orbit to the next.An integer, the quantum number n, is used to designate the orbits.

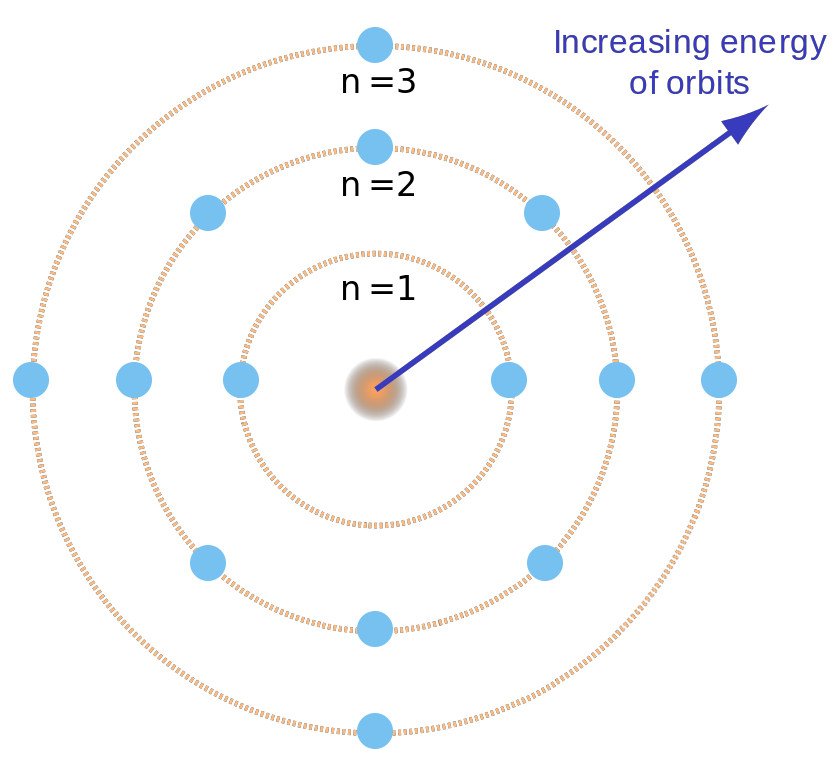
The electrons in an atom gain energy and move from a low energy level to a high energy level, and the electrons lose energy and move from a high energy level to a low energy level. are assigned to the shell and are considered to be in the ground state when the electron reaches the lowest energy level. This quantum number range starts from the core side and has the lowest energy level of n = 1. Quantum numbers are integers (n = 1, 2, 3. These circular orbits are called orbital shells because each orbital or shell has defined energy.
Whenever an electron transitions from one orbit to another, it absorbs or emits radiation.Įlectrons in an atom (negatively charged) rotate in a specific circular path around a positively charged nucleus. The lowest energy is in the smallest orbit. The energy of the orbit is proportional to its size. It orbits around the core in a fixed size, fixed energy orbit. The attractive force of the solar system is mathematically equivalent to the Coulomb force (electric force) that exists between a positively charged nucleus and a negatively charged electron. This is a space model in which negatively charged electrons orbit a small positively charged nucleus, much like a planet orbits the Sun (unless the orbit is non-planar).


 0 kommentar(er)
0 kommentar(er)
