
Then undergo electron pairing during chemical bond formation. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals, which can Then, these four orbitals, the 2s orbital and the three 2p orbitals, mix. What happens is one of the 2s electrons is promoted to a 2p orbital. It would be reasonable to suggest that the 2s and 2p orbitals interact differently Plane-polarized, each around one of the three Cartesian coordinate axes. p-type orbitals are complex dumbbell shapes, which are The remaining two electrons are unpaired and are found in two of the 2p orbitals, The 2s orbital contains two electrons, and this orbital is spherical in shape. The 2s orbital is lower in energy than the 2p orbitals. The four electrons in the second energy level, or second quantum number, areĭistributed between the 2s orbital and the 2p orbitals. Specifically, they are found in an s orbital. The two electrons in the first shell have the lowest energy. And in the second shell or quantum number, there are four electrons. In the first shell or quantum number, there are two electrons. These six electrons are arranged into the different energy levels, or quantum There are six electrons in a carbon atom. Since the number of protons is equal to the number of electrons in a neutral atom, This tells us that an atom of carbon has six protons in its nucleus. Let’s clear some space to see this visually.Ī carbon atom has atomic number Z of six. This is this second energy level of a carbon atom. When referring to orbitals in a quantum number, it is the principal quantum number Orbitals in a hybridized carbon atom in relation to the atomic orbitals in an In this question, we are asked to describe the energy level of the sp3 hybrid (E) The sp3 hybrid orbitals are at an energy level in between the original 2s orbital (D) The sp3 hybrid orbitals are at a lower energy level than the original 2s (C) The sp3 hybrid orbitals are at a higher energy level than the original 2p (B) The sp3 hybrid orbitals are at the same energy level as the original 2s Orbitals? (A) The sp3 hybrid orbitals are at the same energy level as the original 2p Which statement correctly describes the energy level of the resulting sp3 hybrid

hybrid orbitals: The atomic orbitals obtained when two or more nonequivalent orbitals form the same atom combine in preparation for bond formation.Atomic orbitals in the second quantum number of a carbon atom hybridize to form sp3 Hybridization: The mixing of the atomic orbitals in an atom to produce a set of hybrid orbitals. Similarly, the 3p x, 3p y, and 3p z are degenerate orbitals.
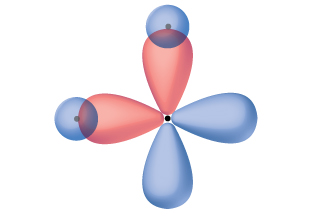
Orbitals in the 2p sublevel are degenerate orbitals – Which means that the 2p x, 2p y, and 2p z orbitals have the exact same energy, as illustrated in the diagram provided below. In the diagram below, the p orbitals have combined, and the π-electrons are delocalized. The p orbitals combine side on and the electrons in the p orbitals are described as π-electrons. The sp 2 hybrid orbitals produce normal covalent bonds, sometimes called σ-bonds: these are the single C-C bonds and single C-H bonds. Now, we have got the complete detailed explanation and answer for everyone, who is interested! This is a question our experts keep getting from time to time. Are hybrid orbitals energetically degenerate?


 0 kommentar(er)
0 kommentar(er)
